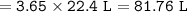Chemistry, 10.03.2022 14:40 NathanaelLopez
If one mol of gas = 22.4 at STP how many liters is occupied by 3.65 moles of oxygen gas at STP

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2
You know the right answer?
If one mol of gas = 22.4 at STP how many liters is occupied by 3.65 moles of oxygen gas at STP...
Questions

Mathematics, 12.03.2021 07:00

Social Studies, 12.03.2021 07:00

English, 12.03.2021 07:00


Mathematics, 12.03.2021 07:00


History, 12.03.2021 07:00

History, 12.03.2021 07:00


Social Studies, 12.03.2021 07:00

Chemistry, 12.03.2021 07:00

Physics, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

Social Studies, 12.03.2021 07:00



Mathematics, 12.03.2021 07:00

Biology, 12.03.2021 07:00