
Chemistry, 12.03.2022 18:30 tahjaybenloss16
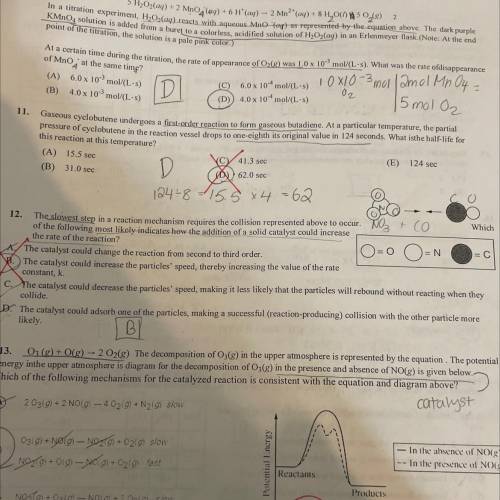
the slowest step in a reaction mechanism requires the collision represented above to occur. Which of the following most likely indicates how the addition of a solid catalyst could increase the rate of the reaction?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

You know the right answer?
the slowest step in a reaction mechanism requires the collision represented above to occur. Which of...
Questions


Chemistry, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10



Mathematics, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10


History, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10


Physics, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10


Mathematics, 02.03.2021 18:10


Chemistry, 02.03.2021 18:10

Mathematics, 02.03.2021 18:10



