
Chemistry, 13.03.2022 01:00 samanthasheets8006
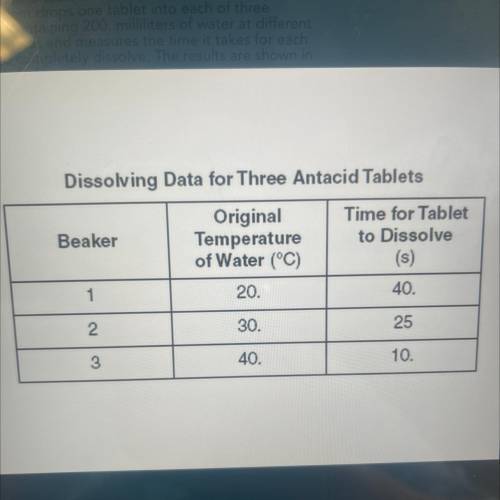
A student conducts an experiment to determine how
the temperature of water affects the rate at which an
antacid tablet dissolves in the water. The student has
three antacid tablets of the same size and composition.
The student drops one tablet into each of three
beakers containing 200. milliliters of water at different
temperatures and measures the time it takes for each
tablet to completely dissolve. The results are shown in
the table below.
10.) What change, other than temperature, would
affect the rate of dissolving?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2

Chemistry, 23.06.2019 17:30
Describe the type of force which exists between particles in an ideal gas. explain why this type of force exists between the particles.
Answers: 3

Chemistry, 24.06.2019 00:00
The total momentum of two marbles before a collision is 0.06 kg-m/s. no outside force act on the marbles. what is the total momentum of the marbles after collision
Answers: 1
You know the right answer?
A student conducts an experiment to determine how
the temperature of water affects the rate at whi...
Questions


English, 15.12.2021 03:50

English, 15.12.2021 03:50

Computers and Technology, 15.12.2021 03:50












Social Studies, 15.12.2021 03:50




Mathematics, 15.12.2021 03:50



