
Chemistry, 15.03.2022 02:50 treymartinez7250
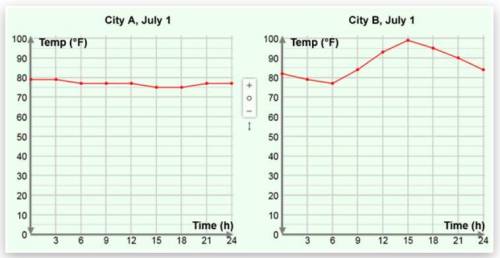
Temperature graphs from two cities on July 1 are shown below. Which statement is true?
A. City A experienced a bigger temperature change than City B.
B. City B experienced a bigger temperature change than City A.
C. The low temperature in City B was lower than the low temperature in City A.
D. Both B and C are true.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 19:30
What are the two most influential greenhouse gases in maintaining the earth's climate? a) carbon dioxide & silicon b) water vapor & carbon dioxide c) silicon & oxygen d) nitrogen & water vapor
Answers: 2

Chemistry, 23.06.2019 20:00
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant r. suppose the osmotic pressure of a certain solution is measured to be 12.atm at an absolute temperature of 317k. write an equation that will let you calculate the molarity c of this solution. your equation should contain only symbols. be sure you define each symbol other than r. equation: c=? definition of symbols: ? = 12 atm ? = 317 k
Answers: 3

Chemistry, 24.06.2019 01:20
What would be the greatest difference in using an open ceramic coffee mug rather than an insulated mug with a lid as a calorimeter? increased evaporation of the water loss of gaseous reactants inability to contain liquids exchange of energy with the surroundings
Answers: 1

Chemistry, 24.06.2019 03:00
The standard gibbs energy of formation of nh3(g) is –16.5 kjmol−1 at 298k. what is the reaction gibbs energy when the partial pressures of the n2, h2, and nh3 (treated as perfect gases) are 3.0bar, 1.0bar, and 4.0bar, respectively? what is the spontaneous direction of the reaction in this case?
Answers: 1
You know the right answer?
Temperature graphs from two cities on July 1 are shown below. Which statement is true?
A. City A e...
Questions

History, 04.07.2019 08:00





Social Studies, 04.07.2019 08:00




Business, 04.07.2019 08:00









Business, 04.07.2019 08:00



