Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
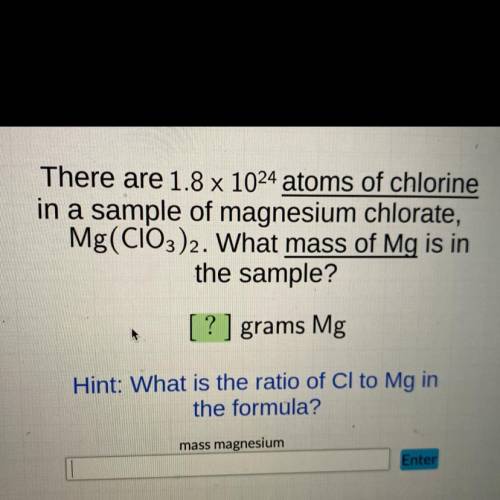
There are 1.8 x 10E24 atoms of chlorine in a sample of magnesium chlorate, Mg (ClO3)2 . What mass of...
Questions


Biology, 03.02.2020 04:48

Mathematics, 03.02.2020 04:48

History, 03.02.2020 04:48

Mathematics, 03.02.2020 04:48




Mathematics, 03.02.2020 04:48

Mathematics, 03.02.2020 04:48

Chemistry, 03.02.2020 04:48


History, 03.02.2020 04:48



Biology, 03.02.2020 04:48

Mathematics, 03.02.2020 04:48



Mathematics, 03.02.2020 04:49