Carbon disulfide is produced by the reaction listed below:
--->
If you started the...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
Questions


Mathematics, 29.11.2021 16:50

Social Studies, 29.11.2021 16:50

Social Studies, 29.11.2021 16:50

History, 29.11.2021 16:50

History, 29.11.2021 16:50



Mathematics, 29.11.2021 16:50


History, 29.11.2021 16:50

Computers and Technology, 29.11.2021 16:50




History, 29.11.2021 16:50

Biology, 29.11.2021 16:50

Mathematics, 29.11.2021 16:50


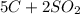 --->
--->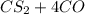
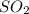 and excess carbon, what amount, in moles, of
and excess carbon, what amount, in moles, of 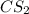 will be produced?
will be produced?

