
Chemistry, 13.04.2022 01:50 tordiacasey
Calculate the enthalpy of reaction for the reaction. C3H8(1) + 502(g) --> 4H2O(g) + 3C02(g) *
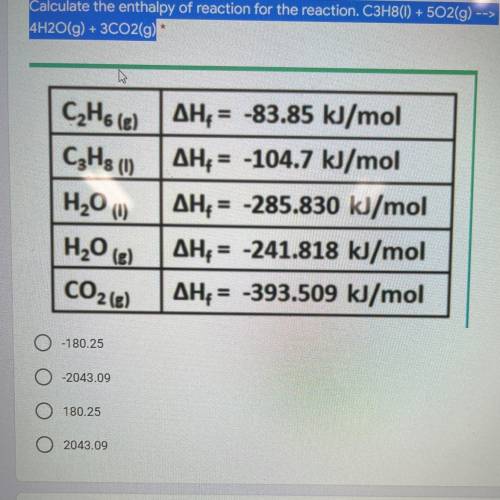

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1
You know the right answer?
Calculate the enthalpy of reaction for the reaction. C3H8(1) + 502(g) --> 4H2O(g) + 3C02(g) *
Questions



Social Studies, 30.05.2021 22:20

Social Studies, 30.05.2021 22:20



English, 30.05.2021 22:20

Mathematics, 30.05.2021 22:20

Mathematics, 30.05.2021 22:20



Mathematics, 30.05.2021 22:20


English, 30.05.2021 22:20

Mathematics, 30.05.2021 22:20


Mathematics, 30.05.2021 22:20



History, 30.05.2021 22:20



