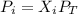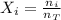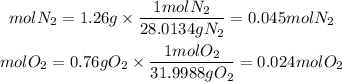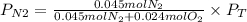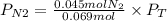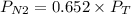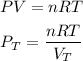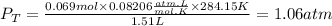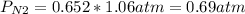Chemistry, 28.01.2023 14:00 itzhari101
A gas mixture contains 1.26 g N2 and 0.76 g O2 in a 1.51 L container at 11 C. Calculate the partial pressure of N2?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 22:30
Which statement best summarizes the importance of ernest rutherford’s gold foil experiment? it proved that all of john dalton’s postulates were true. it verified j. j. thomson’s work on the atomic structure. it showed that an electron circles a nucleus in a fixed-energy orbit. it showed that a nucleus occupies a small part of the whole atom.
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 05:30
Idont understand it 1.what is the boiling point of a solution of 675 grams of ethylene glycol (c2h6o2) in 2.50 liters of water?
Answers: 2
You know the right answer?
A gas mixture contains 1.26 g N2 and 0.76 g O2 in a 1.51 L container at 11 C. Calculate the partial...
Questions

Mathematics, 28.07.2019 12:00



History, 28.07.2019 12:00



Mathematics, 28.07.2019 12:00


Arts, 28.07.2019 12:00





Mathematics, 28.07.2019 12:00

Mathematics, 28.07.2019 12:00




Mathematics, 28.07.2019 12:00