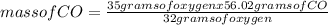When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many grams of co can be formed from 35.0 grams of oxygen? 2c + o2 → 2co using 32.00 g/mole as the molecular mass of oxygen and 28.01 g/mole as the molecular mass of carbon monoxide, solve the above problem.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

You know the right answer?
When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many gram...
Questions





Chemistry, 28.12.2020 17:20





Mathematics, 28.12.2020 17:20



Mathematics, 28.12.2020 17:30







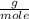 O₂: 32
O₂: 32