

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

You know the right answer?
In the reaction mg(s) + 2hcl(aq) → h2(g) + mgcl2(aq), how many moles of hydrogen gas will be produce...
Questions



Mathematics, 20.09.2020 19:01


Mathematics, 20.09.2020 19:01




Chemistry, 20.09.2020 19:01



Mathematics, 20.09.2020 19:01

Biology, 20.09.2020 19:01


History, 20.09.2020 19:01





Business, 20.09.2020 19:01

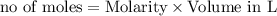
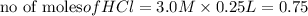
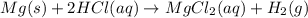
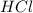 is a limiting reagent as it limits the formation of products and Mg is an excess reagent.
is a limiting reagent as it limits the formation of products and Mg is an excess reagent.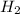
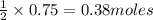 of
of 

