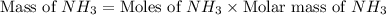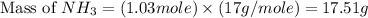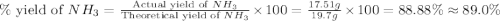Chemistry, 12.10.2019 14:30 jademckinziemea
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent yield of ammonia if the actual yield is 1.03 moles and the theoretical yield is 19.7 grams?
a. 17.5%
b. 35.0%
c. 52.3%
d. 89.0%

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3

Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent...
Questions


Mathematics, 27.01.2020 21:31



Mathematics, 27.01.2020 21:31







History, 27.01.2020 21:31


History, 27.01.2020 21:31

History, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31


Biology, 27.01.2020 21:31

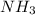 = 1.03 mole
= 1.03 mole