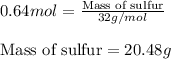Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1



Chemistry, 23.06.2019 08:50
Why are enzymes important to cells? they bring about chemical reactions. they provide structural support. they form the two layers of membranes. they store large quantities of energy.
Answers: 2
You know the right answer?
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: s(g) + 3f2(g) -...
Questions


Social Studies, 28.01.2021 18:50



History, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50

Mathematics, 28.01.2021 18:50



Mathematics, 28.01.2021 18:50

Chemistry, 28.01.2021 18:50


Advanced Placement (AP), 28.01.2021 18:50

Mathematics, 28.01.2021 18:50




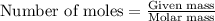 .....(1)
.....(1)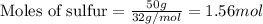
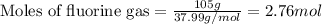
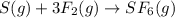
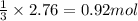 of sulfur
of sulfur