The oxidation state of N in 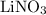 is
is 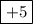
Further Explanation:
Redox reaction:
It is a type of chemical reaction in which the oxidation states of atoms are changed. In this reaction, both reduction and oxidation are carried out at the same time. Such reactions are characterized by the transfer of electrons between the species involved in the reaction.
The process of gain of electrons or the decrease in the oxidation state of the atom is called reduction while that of loss of electrons or the increase in the oxidation number is known as oxidation. In redox reactions, one species lose electrons and the other species gain electrons. The species that lose electrons and itself gets oxidized is called as a reductant or reducing agent. The species that gains electrons and gets reduced is known as an oxidant or oxidizing agent. The presence of a redox pair or redox couple is a must for the redox reaction.
The general representation of a redox reaction is,
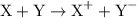
The oxidation half-reaction can be written as:
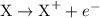
The reduction half-reaction can be written as:

Here, X is getting oxidized and its oxidation state changes from 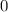 to +1 whereas B is getting reduced and its oxidation state changes from 0 to -1. Hence, X acts as the reducing agent whereas Y is an oxidizing agent.
to +1 whereas B is getting reduced and its oxidation state changes from 0 to -1. Hence, X acts as the reducing agent whereas Y is an oxidizing agent.
Rules to calculate the oxidation states of elements:
1. The oxidation number of a free element is always zero.
2. The oxidation number of oxygen is generally taken as -2, except for peroxides.
3. The oxidation state of hydrogen is normally taken as +1.
4. The sum of oxidation numbers of all the elements present in a neutral compound is zero.
5. The oxidation numbers of group 1 and group 2 elements are +1 and +2 respectively.
The oxidation state of O is -2 and the oxidation state of Li is +1.
The expression to calculate the oxidation state in 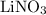 is:
is:
![\left[ {1\left( {{\text{oxidation state of Li}}} \right) + 1\left( {{\text{oxidation state of N}}} \right) + 3\left( {{\text{oxidation state of O}}} \right)} \right] = 0](/tpl/images/0046/1696/e4e42.png) …… (1)
…… (1)
Rearrange equation (1) for the oxidation state of N.
![{\text{Oxidation state of N}} = \left[ { - 1\left( {{\text{oxidation state of Li}}} \right) - 3\left( {{\text{oxidation state of O}}} \right)} \right]](/tpl/images/0046/1696/7b604.png) …… (2)
…… (2)
Substitute -2 for the oxidation  of O and +1 for the oxidation state of Li in equation (2).
of O and +1 for the oxidation state of Li in equation (2).
![\begin{aligned}{\text{Oxidation state}}\;{\text{of N}}&=\left[{-1\left({{\text{+1}}}\right)-3\left({-{\text{2}}}\right)}\right]\\&=\left[{-1+6}\right]\\&=+5\\\end{aligned}](/tpl/images/0046/1696/8a37d.png)
Therefore the oxidation state of N in 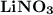 is +5.
is +5.
Learn more:
1. What is the oxidation state of phosphorus in 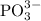 ion?
ion?
2. Determine the oxidation state of Cl in 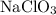 :
:
Answer details:
Grade: High School
Subject: Chemistry
Chapter: Redox reactions
Keywords: redox reaction, N, LiNO3, oxidation, reduction, reductant, oxidant, reducing agent, oxidizing agent, electrons, redox pair, redox couple, oxidation state, oxidized, reduced, simultaneously, +5, -2, +1, Li, O.


























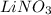 is, (+5)
is, (+5)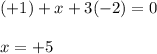
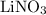 is
is 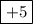
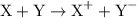
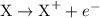

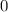 to +1 whereas B is getting reduced and its oxidation state changes from 0 to -1. Hence, X acts as the reducing agent whereas Y is an oxidizing agent.
to +1 whereas B is getting reduced and its oxidation state changes from 0 to -1. Hence, X acts as the reducing agent whereas Y is an oxidizing agent.![\left[ {1\left( {{\text{oxidation state of Li}}} \right) + 1\left( {{\text{oxidation state of N}}} \right) + 3\left( {{\text{oxidation state of O}}} \right)} \right] = 0](/tpl/images/0046/1696/e4e42.png) …… (1)
…… (1)
![{\text{Oxidation state of N}} = \left[ { - 1\left( {{\text{oxidation state of Li}}} \right) - 3\left( {{\text{oxidation state of O}}} \right)} \right]](/tpl/images/0046/1696/7b604.png) …… (2)
…… (2)  of O and +1 for the oxidation state of Li in equation (2).
of O and +1 for the oxidation state of Li in equation (2).![\begin{aligned}{\text{Oxidation state}}\;{\text{of N}}&=\left[{-1\left({{\text{+1}}}\right)-3\left({-{\text{2}}}\right)}\right]\\&=\left[{-1+6}\right]\\&=+5\\\end{aligned}](/tpl/images/0046/1696/8a37d.png)
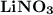 is +5.
is +5.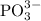 ion?
ion?
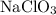 :
:


