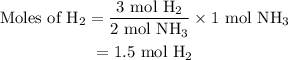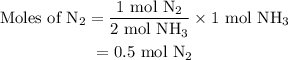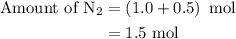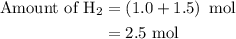Chemistry, 03.07.2019 16:30 campbell387
Amixture of n2(g) and h2(g) reacts in a closed container to form ammonia, nh3(g). the reaction ceases before either reactant has been totally consumed. at this stage 1.0 mol n2, 1.0 mol h2, and 1.0 mol nh3 are present. part a how many moles of n2 and h2 were present originally?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1


Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Amixture of n2(g) and h2(g) reacts in a closed container to form ammonia, nh3(g). the reaction cease...
Questions



Health, 19.09.2019 09:30

Mathematics, 19.09.2019 09:30

Business, 19.09.2019 09:30


Mathematics, 19.09.2019 09:30

Mathematics, 19.09.2019 09:30


History, 19.09.2019 09:30

Social Studies, 19.09.2019 09:30

Biology, 19.09.2019 09:30

Physics, 19.09.2019 09:30




Mathematics, 19.09.2019 09:30

Mathematics, 19.09.2019 09:30


Physics, 19.09.2019 09:30

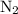 initially taken is
initially taken is 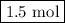 and the amount of
and the amount of 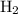 initially taken is
initially taken is 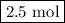 .
.
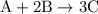
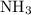 is as follows:
is as follows:
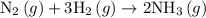
 .
.