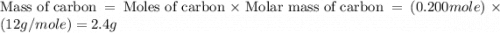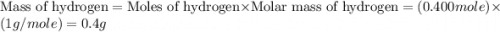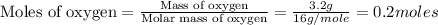Chemistry, 03.07.2019 16:50 naomicervero
If 6.00 g of the unknown compound contained 0.200 mol of c and 0.400 mol of h, how many moles of oxygen, o, were in the sample?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
If 6.00 g of the unknown compound contained 0.200 mol of c and 0.400 mol of h, how many moles of oxy...
Questions

Mathematics, 04.05.2021 06:40

Mathematics, 04.05.2021 06:40




History, 04.05.2021 06:40



English, 04.05.2021 06:40

Social Studies, 04.05.2021 06:40

Mathematics, 04.05.2021 06:40

Chemistry, 04.05.2021 06:40



Chemistry, 04.05.2021 06:40

Mathematics, 04.05.2021 06:40



Mathematics, 04.05.2021 06:40