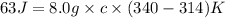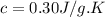Chemistry, 03.07.2019 22:00 jcultr4s3nse
Calculate the specific heat of a substance when 63j of energy are transferred as heat to an 8.0 g sample to raise it temperature from 314 k to 340 k

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 06:00
Complete the sentences to best explain the ranking.match the words below to the appropriate blanks in the sentences.a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
You know the right answer?
Calculate the specific heat of a substance when 63j of energy are transferred as heat to an 8.0 g sa...
Questions



English, 29.09.2021 01:00


Arts, 29.09.2021 01:00

Mathematics, 29.09.2021 01:00


Social Studies, 29.09.2021 01:00


Mathematics, 29.09.2021 01:00

Mathematics, 29.09.2021 01:00

Mathematics, 29.09.2021 01:00



Mathematics, 29.09.2021 01:00


Mathematics, 29.09.2021 01:00



Chemistry, 29.09.2021 01:00

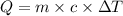
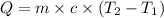
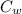 = specific heat of substance = ?
= specific heat of substance = ?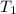 = initial temperature = 314 K
= initial temperature = 314 K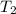 = final temperature = 340 K
= final temperature = 340 K