
Chemistry, 04.07.2019 07:50 jacobmclawhorn3001
Covalent solutes are considered non-electrolytes. what does this mean for the conductivity of the solution? a) non-electrolytes dissolve and completely dissociate in water providing charged ions to conduct electricity. b) non-electrolytes dissolve and do not dissociate in water providing no charged ions to conduct electricity. c) being a non-electrolyte refers the type of bonding and has no bearing on conducting electricity. d) non-electrolytes do not dissolve in water and can not conduct electricity.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1
You know the right answer?
Covalent solutes are considered non-electrolytes. what does this mean for the conductivity of the so...
Questions

Advanced Placement (AP), 23.09.2020 17:01




Mathematics, 23.09.2020 17:01

Mathematics, 23.09.2020 17:01

English, 23.09.2020 17:01


English, 23.09.2020 17:01


Health, 23.09.2020 17:01

English, 23.09.2020 17:01

Chemistry, 23.09.2020 17:01

Mathematics, 23.09.2020 17:01





Mathematics, 23.09.2020 17:01

Mathematics, 23.09.2020 17:01

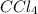 in water will act as a non-electrolyte.
in water will act as a non-electrolyte.

