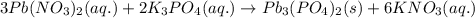Chemistry, 04.07.2019 22:50 natalie407888
When aqueous solutions of lead(ii) nitrate (pb(no3)2) and potassium phosphate (k3po4) are mixed, the products are solid lead(ii) phosphate and aqueous potassium nitrate. write the balanced chemical equation for this reaction. (include states-of-matter under the given conditions in your answer. use the lowest possible whole number

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1


Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
When aqueous solutions of lead(ii) nitrate (pb(no3)2) and potassium phosphate (k3po4) are mixed, the...
Questions




History, 17.10.2019 21:30


Mathematics, 17.10.2019 21:30




English, 17.10.2019 21:30







Mathematics, 17.10.2019 21:30