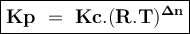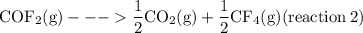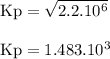Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
The reaction below has an equilibrium constant kp=2.2×106 at 298 k. 2cof2(g)⇌co2(g)+cf4(g) calculate...
Questions


Mathematics, 18.05.2021 04:30



Mathematics, 18.05.2021 04:30





Chemistry, 18.05.2021 04:30


Mathematics, 18.05.2021 04:30



Arts, 18.05.2021 04:30


Arts, 18.05.2021 04:30


Mathematics, 18.05.2021 04:30

History, 18.05.2021 04:30

![\large {\boxed {\bold {Kc ~ = ~ \frac {[C] ^ m [D] ^ n} {[A] ^ p [B] ^ q}}}}](/tpl/images/0052/1661/9d1db.png)
![\large {\boxed {\bold {Kp ~ = ~ \frac {[pC] ^ m [pD] ^ n} {[pA] ^ p [pB] ^ q}}}}](/tpl/images/0052/1661/b3cf6.png)