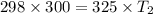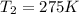Chemistry, 05.07.2019 09:00 natalie2sheffield
The pressure in a car tire is 298 kilopascals at 300 kelvin. after a long drive, the pressure becomes 325 kilopascals. which law will you use to calculate the new temperature in the tire assuming that the volume is constant?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
The pressure in a car tire is 298 kilopascals at 300 kelvin. after a long drive, the pressure become...
Questions

French, 09.11.2020 14:10

Mathematics, 09.11.2020 14:10

Business, 09.11.2020 14:10

Law, 09.11.2020 14:10

Mathematics, 09.11.2020 14:10

Advanced Placement (AP), 09.11.2020 14:10

Biology, 09.11.2020 14:10




Mathematics, 09.11.2020 14:20

English, 09.11.2020 14:20

History, 09.11.2020 14:20

Social Studies, 09.11.2020 14:20

Social Studies, 09.11.2020 14:20

Advanced Placement (AP), 09.11.2020 14:20


Mathematics, 09.11.2020 14:20

Business, 09.11.2020 14:20

English, 09.11.2020 14:20

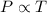 (At constant volume and number of moles)
(At constant volume and number of moles)
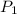 = initial pressure of gas = 298 kPa
= initial pressure of gas = 298 kPa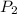 = final pressure of gas = 325 kPa
= final pressure of gas = 325 kPa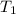 = initial temperature of gas = 300 K
= initial temperature of gas = 300 K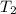 = final temperature of gas = ?
= final temperature of gas = ?