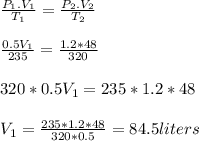Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Ihave an unknown volume of gas at a pressure of 0.5 atm and a temperature of 325k if i raise the pre...
Questions




Mathematics, 29.02.2020 23:05

English, 29.02.2020 23:05

English, 29.02.2020 23:06





Mathematics, 29.02.2020 23:15

Mathematics, 29.02.2020 23:15

Computers and Technology, 29.02.2020 23:15

Mathematics, 29.02.2020 23:15






Mathematics, 29.02.2020 23:16