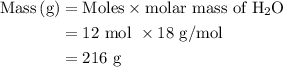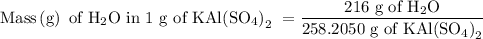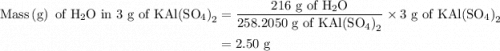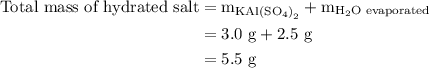Chemistry, 08.07.2019 14:30 christinapim
Suppose harry begins with the hydrate kal(so4)2·12h2o. after dehydration he finds that he is left with 3.0 g of the an-hydrate kal(so4)2. how many grams did he start with?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Suppose harry begins with the hydrate kal(so4)2·12h2o. after dehydration he finds that he is left wi...
Questions


History, 19.02.2020 13:09

English, 19.02.2020 13:09

Chemistry, 19.02.2020 13:10


Chemistry, 19.02.2020 13:11

English, 19.02.2020 13:13

Physics, 19.02.2020 13:16



English, 19.02.2020 13:18

Biology, 19.02.2020 13:18

Mathematics, 19.02.2020 13:19


Mathematics, 19.02.2020 13:26


Chemistry, 19.02.2020 13:28

Geography, 19.02.2020 13:28

History, 19.02.2020 13:29

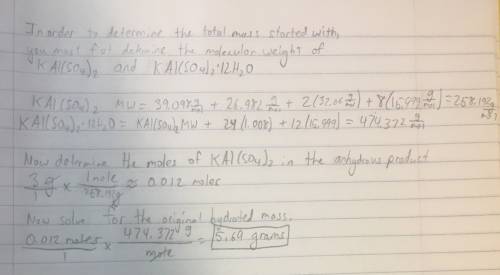
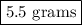 of
of 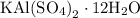 hydrated salt.
hydrated salt.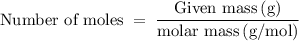
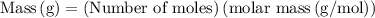
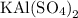 is 258.2050 g/mol.
is 258.2050 g/mol.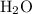 molecules is,
molecules is,