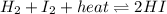Chemistry, 09.07.2019 19:00 tddreviews
Consider the chemical reaction in equilibrium. h2 + i2 + heat ⬄ 2hi what will happen to the chemical equilibrium if the temperature of the system is increased? a)the direction of the chemical equilibrium will shift to the right, favoring the forward reaction. b)the chemical equilibrium will not be affected by an increase in temperature. c)the direction of the chemical equilibrium will shift to the left, favoring the reverse reaction. d) the chemical equilibrium will be lost permanently with a change of temperature.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2

Chemistry, 23.06.2019 07:00
An unknown substance is a white solid at room temperature and has a melting point of 78 °c. which of the following substances is most likely to be the identity of the unknown sample?
Answers: 1
You know the right answer?
Consider the chemical reaction in equilibrium. h2 + i2 + heat ⬄ 2hi what will happen to the chemic...
Questions





Mathematics, 16.11.2020 18:00

Mathematics, 16.11.2020 18:00


Mathematics, 16.11.2020 18:00

Mathematics, 16.11.2020 18:00


Computers and Technology, 16.11.2020 18:00



Spanish, 16.11.2020 18:00


English, 16.11.2020 18:00

Mathematics, 16.11.2020 18:00


English, 16.11.2020 18:00

Biology, 16.11.2020 18:00