Chemistry, 10.07.2019 02:50 lightskinbaby2
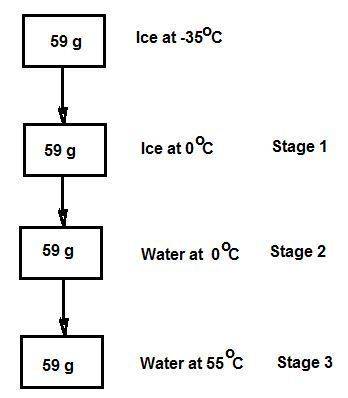
Consider the specific heats h2o(s) = 2.09 j/g · ◦c, h2o (ℓ) = 4.18 j/g · ◦c, and h2o(g) = 2.03 j/g · ◦c. the heat of fusion for water is 334 j/g and its heat of vaporization is 2260 j/g. calculate the amount of heat required to convert 59 g of ice at −35◦c completely to liquid water at 55◦c. answer in units of kj.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Agood hypothesis includes which of the following? a: prediction b: data c: uncertainty d: conclusion
Answers: 1

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
Consider the specific heats h2o(s) = 2.09 j/g · ◦c, h2o (ℓ) = 4.18 j/g · ◦c, and h2o(g) = 2.03 j/g ·...
Questions


Mathematics, 09.04.2021 02:30






Mathematics, 09.04.2021 02:30

Mathematics, 09.04.2021 02:30

Mathematics, 09.04.2021 02:30

Mathematics, 09.04.2021 02:30

English, 09.04.2021 02:30

Biology, 09.04.2021 02:30


Biology, 09.04.2021 02:30

History, 09.04.2021 02:30

History, 09.04.2021 02:30