
Chemistry, 20.08.2019 03:00 dheydar9377
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final reading is 38.55 ml. using the correct number of significant figures, determine the density of the metal = g/ml first determine volume of metal: report result to the same number of decimal places as the number with the least number of decimal places. use this volume in calculation. second determine density of metal: d=m/v report result to the same number of significant figures as the number with the least number of significant figures. do not use exponential notation in answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1


Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
Agraduated cylinder contains 15.75 ml of water. a metal that weighs 31.745 g is added. the final rea...
Questions


Mathematics, 21.04.2020 22:27

Mathematics, 21.04.2020 22:27


Mathematics, 21.04.2020 22:27


Mathematics, 21.04.2020 22:27


English, 21.04.2020 22:27


Business, 21.04.2020 22:27

History, 21.04.2020 22:27

Social Studies, 21.04.2020 22:27

Mathematics, 21.04.2020 22:27

Computers and Technology, 21.04.2020 22:27

Mathematics, 21.04.2020 22:27

Biology, 21.04.2020 22:27

Biology, 21.04.2020 22:27

Mathematics, 21.04.2020 22:27

History, 21.04.2020 22:27

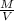 =
= 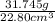 = 1.392 g/cm³
= 1.392 g/cm³

