
Chemistry, 16.09.2019 13:30 Uhmjujiooo4220
Enough of a monoprotic acid is dissolved in water to produce a 0.0116 m solution. the ph of the resulting solution is 2.35. calculate the ka for the acid.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
Enough of a monoprotic acid is dissolved in water to produce a 0.0116 m solution. the ph of the resu...
Questions

Social Studies, 30.01.2020 09:59

History, 30.01.2020 09:59





English, 30.01.2020 09:59


Mathematics, 30.01.2020 09:59


History, 30.01.2020 10:00


Mathematics, 30.01.2020 10:00

English, 30.01.2020 10:00

History, 30.01.2020 10:00

History, 30.01.2020 10:00

History, 30.01.2020 10:00

Social Studies, 30.01.2020 10:00

Biology, 30.01.2020 10:00

Mathematics, 30.01.2020 10:00

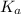 = 1.72 × 10⁻³
= 1.72 × 10⁻³![K_{a} = \frac{[H_{3} O^{+}][ A^{-}] }{[HA]}](/tpl/images/0233/1446/cf7d2.png)
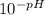 mol/L=
mol/L= 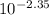 mol/L = 4.47 × 10⁻³ mol/L
mol/L = 4.47 × 10⁻³ mol/L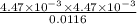 = 1.72 × 10⁻³
= 1.72 × 10⁻³

