Chemistry, 10.07.2019 20:50 shekinahdavis8760
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an atomic mass of 84.912 amu and occurs at an abundance of 72.17%. 87rb has an atomic mass of 86.909 amu and occurs at an abundance of 27.83%. show your work.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 11:00
Surface currents are caused by blank space . question 14 options: surface currents are caused by? differences in water temperature high salinity differences in density wind forces
Answers: 1
You know the right answer?
Calculate the average atomic mass of rubidium. rubidium has two isotopes, 85rb and 87rb. 85rb has an...
Questions

History, 26.06.2019 00:30

Mathematics, 26.06.2019 00:30


Mathematics, 26.06.2019 00:30



Mathematics, 26.06.2019 00:30


Biology, 26.06.2019 00:30



Mathematics, 26.06.2019 00:30



English, 26.06.2019 00:30



Mathematics, 26.06.2019 00:30

Social Studies, 26.06.2019 00:30

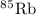 has atomic mass = 84.912 amu
has atomic mass = 84.912 amu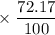
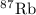 has atomic mass = 86.909 amu
has atomic mass = 86.909 amu