
Chemistry, 20.08.2019 21:30 rfcordray307
For co2 the van der waals constants are a=3.59 atm • l2/mol2 and b=0.0427 l/mol. calculate the pressure exerted by 2.50 moles of co2 confined in a volume of 5.00 l at 450 k.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
For co2 the van der waals constants are a=3.59 atm • l2/mol2 and b=0.0427 l/mol. calculate the press...
Questions

Mathematics, 26.03.2020 22:03

Mathematics, 26.03.2020 22:03






Mathematics, 26.03.2020 22:03

History, 26.03.2020 22:03




Health, 26.03.2020 22:03

Mathematics, 26.03.2020 22:03



Mathematics, 26.03.2020 22:03



Mathematics, 26.03.2020 22:03

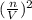 ](V-nb) = nRT;
[P + 3.59 atm·L²mol⁻²
](V-nb) = nRT;
[P + 3.59 atm·L²mol⁻²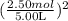 ](5.00 L - 2.50 mol × 0.0427 mol·L⁻¹) = 2.50 mol × 0.08206 L·atm·K⁻¹mol⁻¹ × 450 K;
(P + 0.898 atm)(5.00 L - 0.107 L) = 92.3 L·atm;
(P + 0.898 atm)(4.89) = 92.3 atm;
P + 0.898 atm =
](5.00 L - 2.50 mol × 0.0427 mol·L⁻¹) = 2.50 mol × 0.08206 L·atm·K⁻¹mol⁻¹ × 450 K;
(P + 0.898 atm)(5.00 L - 0.107 L) = 92.3 L·atm;
(P + 0.898 atm)(4.89) = 92.3 atm;
P + 0.898 atm = 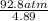 = 18.87 atm;
P = 18.87 atm - 0.898 atm = 17.97 atm
= 18.87 atm;
P = 18.87 atm - 0.898 atm = 17.97 atm

