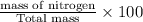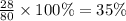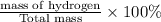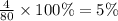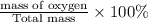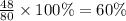The last step is to calculate the percent by mass of each element in ammonium nitrate (nh4no3). the masses of the elements in one mole of the compound are: mass n = 28.0 g mass h = 4.0 g mass o = 48.0 g the molar mass of the compound is 80.0 g/mol. what is the mass of one mole of the compound?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:40
Which are causes of mechanical weathering? check all that apply.oacid raino plant growtho animal actionso carbon dioxideo pressure release
Answers: 1

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

You know the right answer?
The last step is to calculate the percent by mass of each element in ammonium nitrate (nh4no3). the...
Questions

Mathematics, 13.04.2020 20:06



Biology, 13.04.2020 20:06


English, 13.04.2020 20:06





Mathematics, 13.04.2020 20:06

Mathematics, 13.04.2020 20:06


Mathematics, 13.04.2020 20:06






History, 13.04.2020 20:06