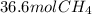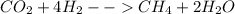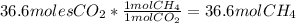Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
How many moles of methane are produced when 36.6 moles of carbon dioxide gas react with excess hydro...
Questions

Mathematics, 27.11.2021 02:30

World Languages, 27.11.2021 02:30


Mathematics, 27.11.2021 02:30

Mathematics, 27.11.2021 02:30


Mathematics, 27.11.2021 02:30

Mathematics, 27.11.2021 02:30


Mathematics, 27.11.2021 02:30

Mathematics, 27.11.2021 02:30


English, 27.11.2021 02:30


English, 27.11.2021 02:30

Advanced Placement (AP), 27.11.2021 02:30



Mathematics, 27.11.2021 02:30