
Chemistry, 12.07.2019 04:30 crosales102
If acetic acid is the only acid that vinegar contains (ka=1.8×10−5), calculate the concentration of acetic acid in the vinegar. express your answer using two significant figures.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2
You know the right answer?
If acetic acid is the only acid that vinegar contains (ka=1.8×10−5), calculate the concentration of...
Questions

Mathematics, 19.02.2020 03:30


Mathematics, 19.02.2020 03:30



Mathematics, 19.02.2020 03:30

Mathematics, 19.02.2020 03:30

Computers and Technology, 19.02.2020 03:30

Mathematics, 19.02.2020 03:30

Social Studies, 19.02.2020 03:30



Mathematics, 19.02.2020 03:30





Mathematics, 19.02.2020 03:31


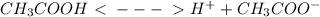
![[H^{+}]](/tpl/images/0079/6240/85507.png) =
= 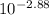 = 0.001
= 0.001 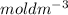
![[CH_{3}COOH]](/tpl/images/0079/6240/d21d8.png) = 0.001
= 0.001  0 0
0 0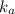 value is so small, the assumption
value is so small, the assumption ![[CH_{3}COOH]_{initial} = [CH_{3}COOH]_{equilibrium}](/tpl/images/0079/6240/01264.png) can be made.
can be made.![k_{a} = [tex]= 1.8*10^{-5} = \frac{[H^{+}][CH_{3}COO^{-}]}{[CH_{3}COOH]} = \frac{0.001^{2}}{x}](/tpl/images/0079/6240/f3563.png)


