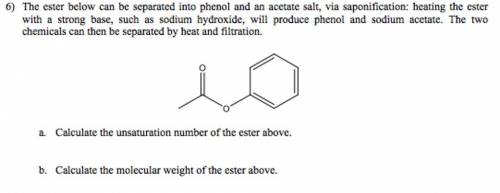
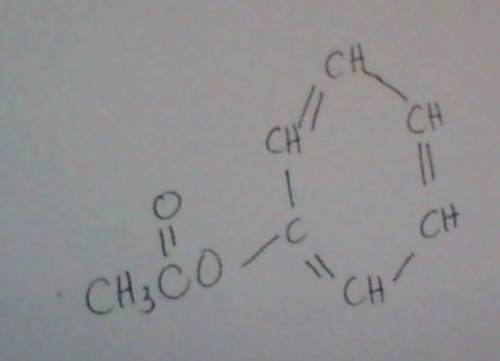
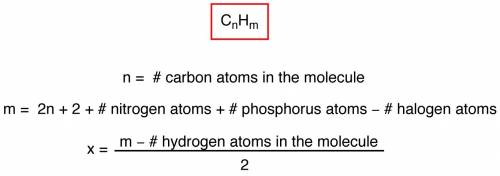
The ester below can be separated into phenol and an acetate salt, via saponification: heating the ester with a strong base, such as sodium hydroxide, will produce phenol and sodium acetate. the two chemicals can then be separated by heat and filtration. a. calculate the unsaturation number of the ester above. b. calculate the molecular weight of the ester above

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
The isotonic saline solution described in part a is connected to an unknown solution via a semipermeable membrane, the unknown solution level drops. based on this information, what can be said about these two solutions?
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3


Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1
You know the right answer?
The ester below can be separated into phenol and an acetate salt, via saponification: heating the e...
Questions

Mathematics, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30




History, 18.07.2019 04:30

Biology, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

Physics, 18.07.2019 04:30


Mathematics, 18.07.2019 04:30

Mathematics, 18.07.2019 04:30

Spanish, 18.07.2019 04:30


History, 18.07.2019 04:30