
Chemistry, 13.07.2019 13:20 wendyyy1214
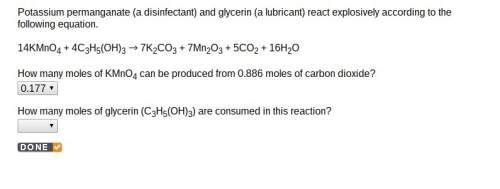
How many moles of glycerin (c3h5(oh)3) are consumed in this reaction? 14kmno4 + 4c3h5(oh)3 es001-1.jpg 7k2co3 + 7mn2o3 + 5co2 + 16h2o

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 23.06.2019 02:00
What causes the appearance of lines in a emission spectrum
Answers: 1
You know the right answer?
How many moles of glycerin (c3h5(oh)3) are consumed in this reaction? 14kmno4 + 4c3h5(oh)3 es001-1....
Questions


History, 24.06.2019 17:20




Mathematics, 24.06.2019 17:20





Mathematics, 24.06.2019 17:20

History, 24.06.2019 17:20

Spanish, 24.06.2019 17:20

Mathematics, 24.06.2019 17:20



Business, 24.06.2019 17:20


Biology, 24.06.2019 17:20

Biology, 24.06.2019 17:20



