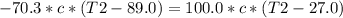Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
A100.0 g sample of water at 27.0°c is poured into a 70.3 g sample of water at 89.0°c. what will be t...
Questions

Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20


English, 10.02.2021 05:20


Advanced Placement (AP), 10.02.2021 05:20


Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20

History, 10.02.2021 05:20

History, 10.02.2021 05:20

History, 10.02.2021 05:20

Mathematics, 10.02.2021 05:20

Social Studies, 10.02.2021 05:20




English, 10.02.2021 05:20

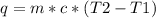 -----(1)
-----(1)