

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 00:00
Mercury turns to a vapor at 629.88 k. how much heat is lost when 75.0 g of mercury vapor at 650 k condenses to a liquid at 297 k?
Answers: 1

Chemistry, 23.06.2019 10:00
Why sncl2 is solid while sncl4 is liquid at room temprature explain it in easy way
Answers: 1

Chemistry, 23.06.2019 13:20
Which nuclide is most likely to be radioactive and synthetic 24/12 mg237/93mg195/78mg230/84mg
Answers: 1
You know the right answer?
A0.8838-g sample of an ionic compound containing bromide ions and an unknown metal cation is dissolv...
Questions





English, 24.06.2019 07:00

Physics, 24.06.2019 07:00

Mathematics, 24.06.2019 07:00

Mathematics, 24.06.2019 07:00

Business, 24.06.2019 07:00

Mathematics, 24.06.2019 07:00

Mathematics, 24.06.2019 07:00

History, 24.06.2019 07:00

History, 24.06.2019 07:00

English, 24.06.2019 07:00


Geography, 24.06.2019 07:00


Geography, 24.06.2019 07:00


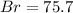 %
%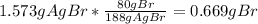
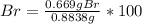 %
%

