
Classify the substances according to the strongest solute-solvent interaction that will occur between the given substances and water during dissolution. match. 1. ion-dipole forces 2. dipole-dipole forces 3. hydrogen bonding 4. london dispersion forces a. alcl3 b. febr3 c. nh3 d. c2h5oh

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

You know the right answer?
Classify the substances according to the strongest solute-solvent interaction that will occur betwee...
Questions



Chemistry, 13.10.2020 05:01

English, 13.10.2020 05:01



Mathematics, 13.10.2020 05:01

English, 13.10.2020 05:01

Mathematics, 13.10.2020 05:01





Computers and Technology, 13.10.2020 05:01

History, 13.10.2020 05:01

English, 13.10.2020 05:01

History, 13.10.2020 05:01


Engineering, 13.10.2020 05:01

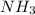 and
and 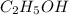 are hydrogen boding,
are hydrogen boding, 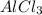 and
and 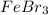 are ion-dipole forces.
are ion-dipole forces.


