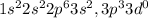Chemistry, 15.07.2019 15:10 angryapple1133
Based on its location in the periodic table, what can most likely be predicted about phosphorus? a. it has three valence electrons available for bonding. b. it has three nonbonding electrons. c. it has five valence electrons available for bonding. d. it has five nonbonding electrons.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

You know the right answer?
Based on its location in the periodic table, what can most likely be predicted about phosphorus? a....
Questions



Mathematics, 09.07.2019 17:00

Social Studies, 09.07.2019 17:00

Biology, 09.07.2019 17:00

Social Studies, 09.07.2019 17:00

Biology, 09.07.2019 17:00



Biology, 09.07.2019 17:00

Mathematics, 09.07.2019 17:00




History, 09.07.2019 17:00


English, 09.07.2019 17:00



Mathematics, 09.07.2019 17:00