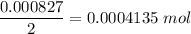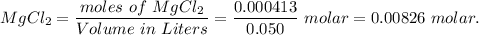When 50.0 ml of a 0.3000 m agno3 solution is added to 50.0 ml of a solution of mgcl2, an agcl precipitate forms immediately. the precipitate is then filtered from the solution, dried, and weighed. if the recovered agcl is found to have a mass of 0.1183 g, what as the concentration of magnesium ions in the original mgcl2 solution?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
When 50.0 ml of a 0.3000 m agno3 solution is added to 50.0 ml of a solution of mgcl2, an agcl precip...
Questions



World Languages, 09.02.2021 06:40



Health, 09.02.2021 06:40


History, 09.02.2021 06:40

Chemistry, 09.02.2021 06:40

Mathematics, 09.02.2021 06:40

Mathematics, 09.02.2021 06:40

English, 09.02.2021 06:40



Mathematics, 09.02.2021 06:40


Law, 09.02.2021 06:40

Mathematics, 09.02.2021 06:40

Health, 09.02.2021 06:40

Mathematics, 09.02.2021 06:40

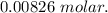
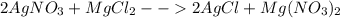
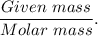
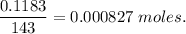 ( Molar mass of AgCl is 143 gm)
( Molar mass of AgCl is 143 gm)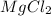 forms 2 mol of AgCl.
forms 2 mol of AgCl.