Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 11:30
If this sedimentary rock layer is truly the oldest one of marine origin, what do you think that tells usabout the formation of earth's oceans?
Answers: 2

Chemistry, 23.06.2019 15:40
Which functions of water in living systems would still be possible if water was not polar and did not form hydrogen bonds? check all that apply. climate regulation dissolving ionic compounds for biological reactions providing body support by exerting pressure on cell walls providing body support through buoyancy transport of nutrients within organisms temperature regulation in many organisms
Answers: 3
You know the right answer?
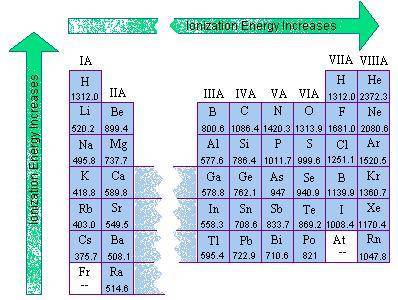
As element of group 1 on the periodic table are considered in order of increasing atomic radius, the...
Questions




History, 25.11.2021 05:40

Mathematics, 25.11.2021 05:40










English, 25.11.2021 05:40

Social Studies, 25.11.2021 05:40

Mathematics, 25.11.2021 05:40

Social Studies, 25.11.2021 05:40