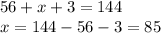Chemistry, 17.07.2019 08:00 rosebelll4555
After the fission of u-235 takes place, how many neutrons does the missing nucleus have? a) 84 b) 85 c) 141 d) 143 the atomic number is consider a unique atom and element identifier. describe the associated subatomic particle, and how it can be used to identify the atom. a) the number of electrons changes in a unique way as the charge on the atom changes. b) the proton number remains constant through changing the atomic charge, energy, and isotopes. c) as the atom absorbs photons the way the photons interact in the nucleus gives the atomic number. d) all isotopes of the an element have the same number of neutrons allowing it to describe the unique atom.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 15:00
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 00:30
The footprints of a dinosaur and the burrow of an ancient shrimp are examples of which kind of fossils
Answers: 2
You know the right answer?
After the fission of u-235 takes place, how many neutrons does the missing nucleus have? a) 84 b)...
Questions

English, 19.01.2021 20:30


Mathematics, 19.01.2021 20:30



Health, 19.01.2021 20:30

Mathematics, 19.01.2021 20:30

Computers and Technology, 19.01.2021 20:30


Mathematics, 19.01.2021 20:30

Physics, 19.01.2021 20:30


Social Studies, 19.01.2021 20:30

Mathematics, 19.01.2021 20:30

Social Studies, 19.01.2021 20:30

Mathematics, 19.01.2021 20:30

English, 19.01.2021 20:30

Geography, 19.01.2021 20:30


Mathematics, 19.01.2021 20:30