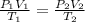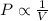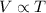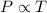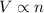Chemistry, 17.07.2019 12:50 nightwolf8999
The table below shows a comparison of the different gas laws. some cells have been left blank. name variables constants equation boyle's law pressure, volume ? pv = k charles’s law volume, temperature ? v = kt ? temperature, pressure volume, moles of gas p = kt ? pressure, temperature, volume ? mc022-1.jpg which are assumed to be constant while using the combined gas law? a. pressure b. number of moles c. volume and moles of gas d. pressure and temperature

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
The table below shows a comparison of the different gas laws. some cells have been left blank. name...
Questions





Biology, 05.02.2021 21:20

Mathematics, 05.02.2021 21:20

Mathematics, 05.02.2021 21:20


History, 05.02.2021 21:20

Mathematics, 05.02.2021 21:20








Mathematics, 05.02.2021 21:20