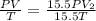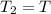Chemistry, 18.07.2019 17:40 ernestscott406
Consider a balloon that has a volume v. it contains n moles of gas, it has an internal pressure of p, and its temperature is t. if the balloon is heated to a temperature of 15.5t while it is placed under a high pressure of 15.5p, how does the volume of the balloon change?

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3
You know the right answer?
Consider a balloon that has a volume v. it contains n moles of gas, it has an internal pressure of p...
Questions



Mathematics, 23.04.2021 01:40

Mathematics, 23.04.2021 01:40

History, 23.04.2021 01:40

Computers and Technology, 23.04.2021 01:40

Physics, 23.04.2021 01:40




Mathematics, 23.04.2021 01:40



SAT, 23.04.2021 01:40



Business, 23.04.2021 01:40


Social Studies, 23.04.2021 01:40

Mathematics, 23.04.2021 01:40

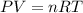
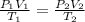
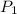 = initial pressure
= initial pressure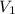 = initial volume
= initial volume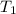 = initial temperature
= initial temperature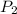 = final pressure
= final pressure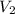 = final volume
= final volume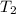 = final temperature
= final temperature