Chemistry, 19.07.2019 04:50 lola06032003
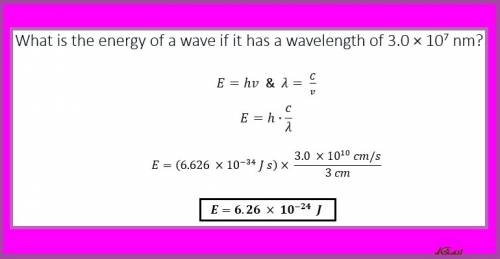
Using c=λν and e=hν (where h or planck’s constant is 6.626 *10 ⁻³⁴js). find the energy that a wavelength has if it has a wavelength of 3.000*10⁷ nm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

You know the right answer?
Using c=λν and e=hν (where h or planck’s constant is 6.626 *10 ⁻³⁴js). find the energy that a wavel...
Questions

Mathematics, 14.12.2021 19:00




Mathematics, 14.12.2021 19:00


Physics, 14.12.2021 19:00


Biology, 14.12.2021 19:00





Mathematics, 14.12.2021 19:00

Mathematics, 14.12.2021 19:00


Mathematics, 14.12.2021 19:00


Advanced Placement (AP), 14.12.2021 19:00