Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
what is the energy of a photon of infrared radiation with a frequency of 2.53 × 1012 hz?...
Questions


Computers and Technology, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

SAT, 20.10.2020 04:01

History, 20.10.2020 04:01



English, 20.10.2020 04:01





Mathematics, 20.10.2020 04:01

Social Studies, 20.10.2020 04:01

Business, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

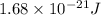
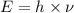
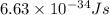
 = frequency of a photon =
= frequency of a photon =