

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
The equilibrium constant is given for one of the reactions below. determine the value of the missing...
Questions



Mathematics, 16.03.2020 16:58






Engineering, 16.03.2020 16:59









History, 16.03.2020 16:59


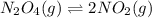
![Kc=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0108/5157/7c0a3.png)
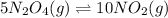
![Kc^'=\frac{[NO_2]^1^0}{[N_2O_4]^5}](/tpl/images/0108/5157/10de9.png)
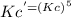
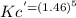
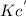 = 6.63
= 6.63

