
Aqueous hydrobromic acid hbr will react with solid sodium hydroxide naoh to produce aqueous sodium bromide nabr and liquid water h2o . suppose 20.2 g of hydrobromic acid is mixed with 5.7 g of sodium hydroxide. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 2 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 21.06.2019 21:30
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2
You know the right answer?
Aqueous hydrobromic acid hbr will react with solid sodium hydroxide naoh to produce aqueous sodium b...
Questions








Biology, 09.10.2020 07:01

Computers and Technology, 09.10.2020 07:01




Health, 09.10.2020 07:01


History, 09.10.2020 07:01

History, 09.10.2020 07:01

Computers and Technology, 09.10.2020 07:01

Mathematics, 09.10.2020 07:01


English, 09.10.2020 07:01

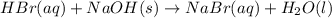
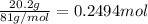
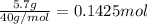
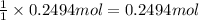 of NaOH.
of NaOH.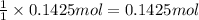 of HBr.
of HBr.

