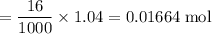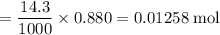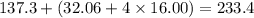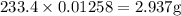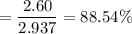Chemistry, 20.07.2019 11:10 isabelcasillas
A16.0 ml sample of a 1.04 m potassium sulfate solution is mixed with 14.3 ml of a 0.880 m barium nitrate solution and this precipitation reaction occurs: k 2 s o 4 (aq)+ba(n o 3 ) 2 (aq)→bas o 4 (s)+2kn o 3 (aq) the solid bas o 4 is collected, dried, and found to have a mass of 2.60 g . determine the limiting reactant, the theoretical yield, and the percent yield.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
A16.0 ml sample of a 1.04 m potassium sulfate solution is mixed with 14.3 ml of a 0.880 m barium nit...
Questions

Physics, 11.02.2021 02:30


Mathematics, 11.02.2021 02:30

Mathematics, 11.02.2021 02:30



Engineering, 11.02.2021 02:30



Mathematics, 11.02.2021 02:30

Mathematics, 11.02.2021 02:30






Health, 11.02.2021 02:30


Mathematics, 11.02.2021 02:30

Social Studies, 11.02.2021 02:30