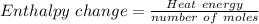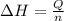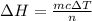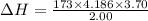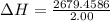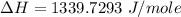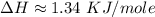Chemistry, 20.07.2019 13:20 thomasdianne
Atotal of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reaction produces 173 g of solution. the reaction caused the temperature of the solution to rise from 21.00 to 24.70 ∘c. what is the enthalpy of this reaction? assume that no heat is lost to the surroundings or to the coffee cup itself and that the specific heat of the solution is the same as that of pure water. enter your answer in kilojoules per mole of compound to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1
You know the right answer?
Atotal of 2.00 mol of a compound is allowed to react with water in a foam coffee cup and the reactio...
Questions

History, 04.06.2020 14:58



Mathematics, 04.06.2020 14:58


English, 04.06.2020 14:58









Medicine, 04.06.2020 14:58

Mathematics, 04.06.2020 14:58