
Chemistry, 20.07.2019 15:10 Benysod402
The main assumptions of the kinetic molecular theory of gases are: 1. gases are made up of molecules which are relatively far apart. 2. the molecules are in motion at high speeds. 3. the molecules are perfectly elastic. 4. increase in temperature increases the kinetic energy of the molecules. the assumption that explains charles' law is: 1 2 3 4 1 and 3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3


Chemistry, 23.06.2019 02:00
The plant food contains nh4)3po4 what tests would you run to verify the presence of the nh4 ion and the po4 ion
Answers: 2
You know the right answer?
The main assumptions of the kinetic molecular theory of gases are: 1. gases are made up of molecule...
Questions

English, 02.12.2020 20:40

Mathematics, 02.12.2020 20:40

Social Studies, 02.12.2020 20:40

Geography, 02.12.2020 20:40

Mathematics, 02.12.2020 20:40

History, 02.12.2020 20:40



Mathematics, 02.12.2020 20:40

Chemistry, 02.12.2020 20:40


Social Studies, 02.12.2020 20:50

Mathematics, 02.12.2020 20:50


English, 02.12.2020 20:50

History, 02.12.2020 20:50



Mathematics, 02.12.2020 20:50

Medicine, 02.12.2020 20:50

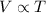 (at constant pressure)
(at constant pressure)

