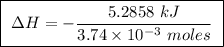Chemistry, 22.07.2019 18:00 ttwright24
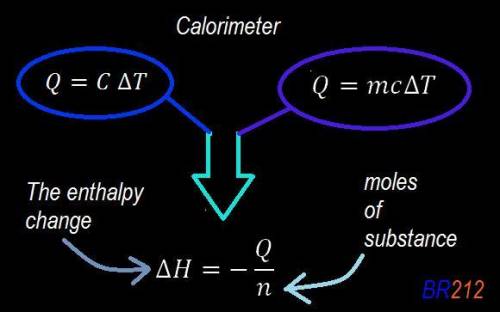
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –0.259 kj/mol b. –50.3 kj/mol c. –5.29 kj/mol d. –1.41 × 103 kj/mol e. –660 kj/mol

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions




Mathematics, 13.09.2021 02:10

Mathematics, 13.09.2021 02:10


Mathematics, 13.09.2021 02:10

Mathematics, 13.09.2021 02:10

Mathematics, 13.09.2021 02:10



Biology, 13.09.2021 02:10


Mathematics, 13.09.2021 02:10

Mathematics, 13.09.2021 02:10


Physics, 13.09.2021 02:10


Biology, 13.09.2021 02:20

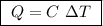
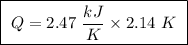
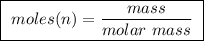
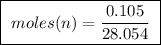
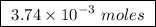 of ethylene.
of ethylene.